Improved Insertion Device for Central-line Insertion Process
Background
Every year, millions of central venous catheters are placed in patients of the U.S. healthcare system. These central lines deliver life-saving medications and fluids.
Many hospitalized patients receive temporary central lines in the form of non-tunneled central venous catheters at one of four insertion sites: internal jugular vein, subclavian vein, peripheral vein, and femoral vein. Our team is addressing the insertion process of a central line in an internal jugular vein.

Needs Statement
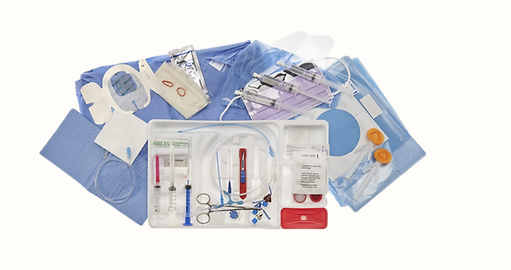
Insertion of a central line requires a clinical provider to follow a lengthy procedure for proper insertion and functioning with many tools in kits. They need to reduce the number of tools used to fasten this procedure and reduce the risk of infection by decreasing the number of touch points during procedures.
Product Development
This product focuses on combining the insertion needle, guidewire and dilator steps of the insertion process into one. We are applying for the patent so I can't show the details CAD due to Public Disclosure. But I can tell who we come up with the design of insertion device simplifies the IV insertion procress.

My teammates identified the physician's needs at DH 1 in the first semester. I joined the team in the second semester. We translated the identified needs into various concepts and potential solutions. By using tools from quality management systems and business models, we systematically evaluated and narrowed down the solutions to a single, viable option. From there, we developed initial prototypes to begin testing and refining the design.
In the third and final semester of the Design Health program, we refined the device prototype based on clinical feedback and developed a more advanced, realistic version for manufacturing. Alongside this, our team crafted a comprehensive regulatory strategy, development timeline, and business plan that could be executed if the device were to be commercialized.
Final Output

We experenced the whole development process from background research, need statement, solution landscape to designing and doing killer experiments, manufacturing, IP research, business strategy. I learnt way more than only from engineering aspect, but also from the management and IP aspects. We have applied for the patent and it is under review now.
Create Your Own Website With Webador